Energy
Basic physics
Every physical system embodies a quantity which is designated as "energy content" or shortly as "energy". Energy is measured in units of Joule (J). Its quantity has additive characteristics: Supply of energy to any system augments, and withdrawal of it reduces its value. This means that the energy in a system can be piled up or given off. Energy is subjected to a physical law of conservation: Within a closed system the total amount of energy is constant. Decrease of energy within one part of the system must correspond to an equivalent increase of energy in the remaining parts of the system. In the same way the increase of energy within one particular sector of the system means that it has been withdrawn from other system sectors. The exchange of energy between different parts of the whole system may take place via different kinds of energy. The most important of them are: mechanical energy/work (M), heat energy (H), chemical energy (C) as well as the energy of electro-magnetic radiation (R) [1, 2, 3]. These different kinds or types of energy can be mutually converted. However, how efficient it can be converted to another form of energy, depends very much on the particular type of energy. For example: The mechanical energy (M) of a rotating wheel can be converted via a dynamo partially into light (R). Or, by mechanical friction, brakes convert mechanical energy (M) completely into heat energy (H). The chemical energy of fuels (C) can be converted into the heat energy of water vapour (H), which again via a turbine converts it to mechanical energy (M). It is a fundamental physical law, that by periodic circular processes like they take place in turbines, heat energy cannot be converted completely into other kinds of energy (see conversion efficiency).
The term power, P, describes the energy flow between two parts of a system. Power is the exchanged energy E divided by the time t which is needed for this exchange: P = E/t. Depending on the type of exchanged energy it is designated as power of work, as heat flow or radiation intensity, where the two last terms are related to the area of energy exchange. Power or energy per time is measured by the unit Watt (W). After time t with power P the energy E = P·t has been exchanged (added to or withdrawn from the system). Therefore, the unit of energy is expressed also by Ws (= J). Another very common unit of energy is kWh which is well known from invoices for electical energy consumption. Table 1 presents an overview of units and conversions thereof.
|
||||
| Table 1: Units of energy and power. |
 |
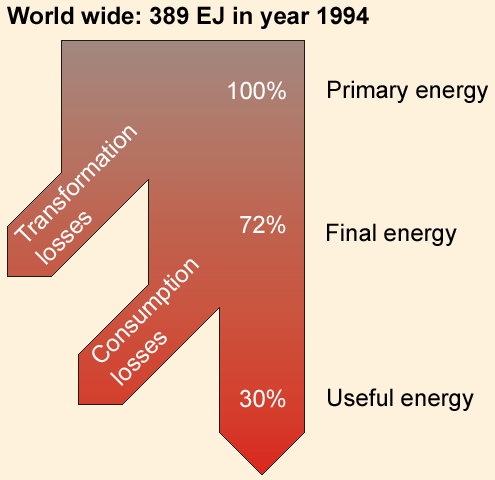
| Figure 1: Energy flow for world-wide conversion losses from primary energy to useful enery in 1994 [4]. |
Fundamental terms of energy technology
Primary energy (raw energy) is the energy content of not yet refined energy sources. They are divided into fossil fuels (oil, gas, coal), nuclear fuels (uranium, thorium) and renewable or regenerative ressources like sun, biomass, wind and geothermal energy.
Final energy is the amount of energy which is available to generate useful energy. During the processes needed to prepare and to convert primary energy the available energy content is reduced (see conversion loss). Typical kinds of final energy are electricity, gasoline, oil for heating and district heat. A large contribution to the energy losses when producing final energy comes from the conversion of heat to electricity (see Table 2).
|
||||||||||||||||||||||||||||||||
| 1) 30 % current plus 55 %
heat (in winter). 2) At a wind velocity higher than 30 km/h. 3) Without energy for production of H2 and O2. |
||||||||||||||||||||||||||||||||
| Table 2: Maxima of obtainable energy efficiencies for different plants, devices and end energy users (estimated upper limits). |
Useful energy is that kind of energy which is really needed. It is different from final energy because there is still a conversion process between final to useful energy. Useful energy means heat for room heating, process heat, mechanical or electrical/electro-magetic energy. Normally this kind of energy is generated from final energy instantly at the time when it is needed.
Energy demand is the expected amount of energy required to generate the needed amount of useful energy from one or several kinds of energy suppliers by the application of appropriate technologies.
Energy consumption is the actually consumed amount of energy for a certain kind of energy, which was required to supply the energy demand.
Energy loss is that part of energy, which, supplied to an existing system, leaves the system without use (with regard to the intended purpose). Especially when converting energy fuels into electricity/mechanical energy via any thermodynamic circular process, only 1/3 up to 1/2 of the primary energy is converted into final energy. The conversion factor depends very much on the temperatures, which are reached during the conversion process. This factor can be significantly improved, if the additional use of heat is technically feasible (co-generation of electricity and heat, see heating systems).
Calorific values (upper, lower) define the released heat energy per burned mass of energy fuel depending on whether the water vapor in the flue gas can be condensed or not. The lower calorific value does not consider any energy gain from condensing water vapor. It only includes the energy gain from burning. If energy gains from condensing flue gas are realized by the technical system (upper value), they are increased by the heat gain due to the condensation of water vapor, which is for about 25°C, nearly 2440 kJ/kg. For solid or fluid fuels the energy content is referred to the mass of 1 kg fuel; for gaseous fuels the reference is 1 m³ of gas under normal conditions (0°C; 1,013 bar). Table 3 presents both calorific values for several sources of primary energy. The lower calorific value of oil is about 10 kWh/liter. This allows a very easy way to describe the energy content of a volume of fossil fuel (oil) in terms of final energy.
|
||||||||||||||||||||||||
| Table 3: Upper and lower calorific values of different fuels. |
Sources of energy and energy demand
For describing the world energy consumption the unit Exa-Joule is appropriate (Exa = 1018):
1 EJ =1018 J = 1 000 000 000 000 000 000 J = 278 billions kWh.
Current (year 2000) annual world consumption of primary energy amounts about 400 EJ. For comparison: The big earth quake in San Francisco from 1906 released an energy of only about 0.05 EJ [3].
Another (at least for Germany) quite common unit of primary energy is SKE (= Steinkohle-Einheit). Usually, it is given in tons of coal (t SKE) and corresponds to the mean lower calorific value of one ton (= 1.000 kg) of coal: 1 t SKE = 8,140 kWh. For the international use more applicable may be the unit Mtoe (millions tons of oil equivalent), where 1 toe is equal to 11,630 kWh. From Figure 2 as well as Tables 1 and 2 can be seen, that the world energy consumption in 1994 (= 389 EJ) was about 13.3 billions t SKE. 300 EJ were contributed by fossile fuels, 24 EJ by nuclear fuels and 65 EJ by renewable energy sources (see renewable energies). Figure 2 presents these contributions in more detail.
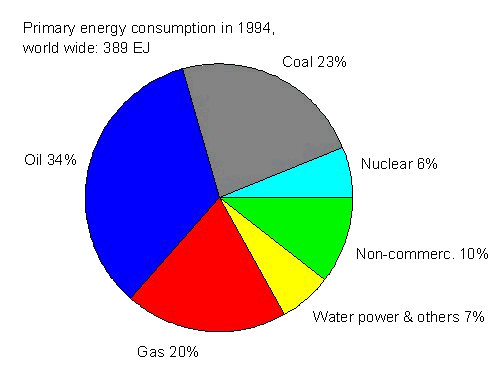
|
| Figure 2: Distribution of world-wide consumption of primary energy in 1994 with respect to coal, oil, natural gas, nuclear energy, hydro and other renewable energy resources as well as non-commercial energies (e.g. wood as biomass, [4]). |
Since 1900 the world´s consumption of primary energy raises strongly and - besides during both world wars and the world economy crisis - continuously up to now (Figure 3). Whereas in 1930 the annual energy consumption was still at 60 EJ, one expects for 2040 an annual consumption between 500 EJ and 600 EJ [4].
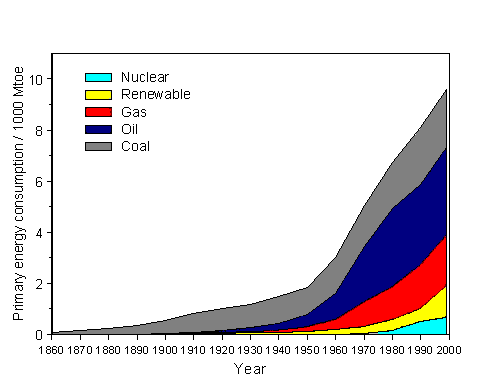 |
| Figure 3: Temporal development of world-wide consumption of primary energy in billions of tons SKE since 1860 for different energy fuels but without consideration of non-commercial energy resources (e.g. wood) according to [4], [7] and [8]. Renewable energies are water, biomass, wind, geothermal and solar energy. |
Most renewable energies are either directly (e.g. solar collector) or indirectly (e.g. wind, biomass) feeded by the sun (see renewable energies). Annually about 2,500,000 EJ of solar energy enter the earth´s surface [5]. This is roughly 6,400 times the primary energy demand which was needed in 1994 world-wide. In other words: To completely cover the current energy demand of the world, only about 0.16‰ of the available solar energy on the earth have to be used.
Characteristic values for energy use
The specific energy consumption (depending on kind of use) is one of the most important characteristic values for the evaluation and qualification of equipment, plants or buildings. One example from "transportation and traffic" is fuel consumption in liters fuel per 100 km of distance. By means of Table 2 this fuel consumption can be converted to specific energy consumption. For example: A consumption of 5 liters (Diesel/oil) per 100 km results in a specific energy consumption of 0.5 kWh/km.
For buildings one uses the specific consumption of heat energy, where "specific" relates to heated floor area. Therefore, the corresponding unit is kWh/(m²·a). For the existing building stock in Germany typical values range between 100 kWh/(m²·a) and 300 kWh/(m²·a). By appropriate measures these values can be drastically reduced for new as well as for the existing buildings (see energy and building).
The efficiency 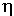 of a system is the ratio of the useful power delivered by the system and the
corresponding supporting power required by the system. For a machine which converts
heat to mechanical energy or electricity, the theoretically maximum obtainable
efficiency depends on the temperature levels between which mechanical work is
realized. The greater the temperature is where heat is added to the system and
the lower it is where heat can be removed, the better is the efficiency of the
conversion process. Table 2 lists examples of equipment, plants and devices
with their efficiencies (typical, or depending on conditions).
of a system is the ratio of the useful power delivered by the system and the
corresponding supporting power required by the system. For a machine which converts
heat to mechanical energy or electricity, the theoretically maximum obtainable
efficiency depends on the temperature levels between which mechanical work is
realized. The greater the temperature is where heat is added to the system and
the lower it is where heat can be removed, the better is the efficiency of the
conversion process. Table 2 lists examples of equipment, plants and devices
with their efficiencies (typical, or depending on conditions).
The efficiency of utilization 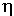 ´
of a system is the ratio of the useful energy delivered by the system and the
corresponding supplied energy required by the system (including the auxiliary
energies). The considered period of time includes all pauses as well as phases
of starting and after ending the operation of the system. Therefore, the efficiency
of utilization
´
of a system is the ratio of the useful energy delivered by the system and the
corresponding supplied energy required by the system (including the auxiliary
energies). The considered period of time includes all pauses as well as phases
of starting and after ending the operation of the system. Therefore, the efficiency
of utilization 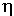 ´ of a
system is always lower than its efficiency
´ of a
system is always lower than its efficiency 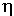 .
.
Cumulated energy demand (CED), measured in J or Wh, is the total sum of primary energy which was needed to produce, operate and recycle any product [6]. When assuming the total life time of a building and its components to about 50 years, the annual values of specific energy demand are situated between 200 kWh/(m²·a) and 400 kWh/(m²·a) or below.
The energy pay back time indicates after which time of operation the system will have paid back the amount of energy which was needed to produce the system, to operate and to remove it. As an example: Photovoltaic plants with cells of amorphous silicium have an energy pay back time between 2 and 4 years; solar thermal plants lie between 1 and 2 years. If the pay back time for energy exceeds the physical life time of a plant, no net saving of resources will be achieved.
Characteristic values of energy-related emissions
Any conversion of energy (electrical, thermal) in plants for electric power or for heat (or both) releases CO2, sulphuric and nitrous oxides, dust and other substances which are harmful for the environment or the climate. Therefore, energy saving is equivalent to environmental and climate protection. How much of the individual materials is released depends on the corresponding fuels, because the ratio of carbon to hydrogen is different for each fuel. Figure 4 presents typical values for CO2-emissions. The diagram, however does not include electrical energy. There, emissions depend very much on the kind and technological status of the individual power plant. For the German mix of power plants (nuclear, coal, natural gas, others) a value of 0.8 kg CO2/ kWh electrical energy can be assumed.
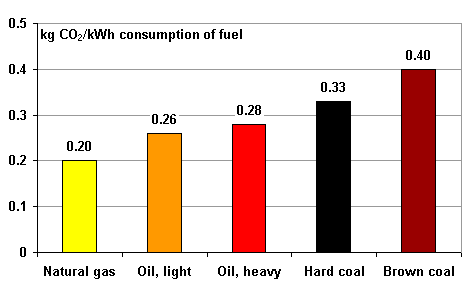 |
| Figure 4: Specific emissions of CO2 for different fuels. |
References
[1] Verein Deutscher Ingenieure (VDI), Energiekennwerte - Definitionen, Begriffe, Methodik - Specific numbers for energy assessment, Definitions - Therms - Methodology, VDI Richtlinie 4661, Beuth Verlag, Berlin, 2000.
[2] Bergmann, L., Schäfer, C.: Lehrbuch der Experimentalphysik, Band 1, Walter de Gruyter, Berlin 1990.
[3] Halliday, D., Resnik, R.: Fundamentals of Physics, John Wiley & Sons, New York, 1988.
[4] Heinloth, K.: Die Energiefrage, Vieweg, Wiesbaden, 1997.
[5] Rabl, A.: Active Solar Collectors and Their Applications, Oxford University Press, Oxford, 1985.
[6] Verein Deutscher Ingenieure (VDI): Kumulierter Energieaufwand - Begriffe, Definitionen, Berechnungsmethoden - Cumulated energy demand - Terms, definitions, methods of calclation, VDI Richtlinie.
[7] Schilling, H.-D., Hildebrandt, R., Die Entwicklung des Verbrauchs an Energieträgern und an elektrischer Energie in der Welt, in den USA und in Deutschland seit 1860 bzw. 1925, in: Rohstoffwirtschaft International, Band 6, Hrg.: W. Peters, Verlag Glückauf GMBH, Essen, 1977.
[8] International Energy Agency (IEA): Key World Energy Statistics, 2002, http://www.iea.org/statist/keyworld/keystats.htm